Aluminium is a base metal and it immediately oxidizes when it comes into contact with air. From a chemical point of view, the formed oxide layer is more stable than aluminium itself and this is the key to the corrosion resistance of aluminium. However, the effectiveness of this layer can also be diminished – by alloying elements, for example. This is what you need to know.
For applications where visual appearance is not critical, the natural oxide layer may offer sufficient corrosion protection. But if the aluminium is to be painted, bonded, or used in a corrosive environment, a pre-treatment is necessary to create a more stable and well-defined surface. The composition of aluminium oxide layers can vary, depending on formation conditions, alloying elements, and contaminants. When water is present during oxidation, crystal water may also be present in the oxide layer. The stability of the oxide layer is influenced by its composition.
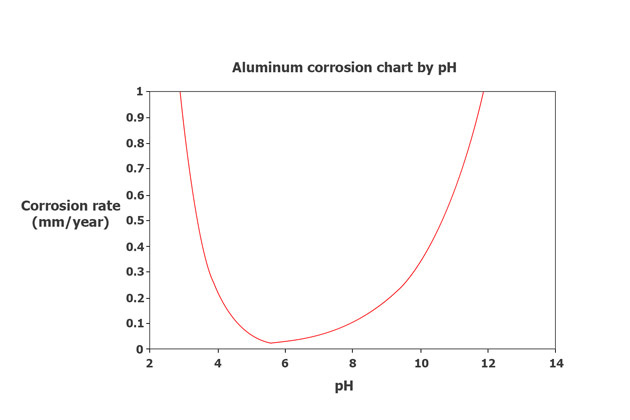
Aluminium oxide is typically stable within a pH range of 4 to 9. Outside of this range, the risk of corrosion is higher. Consequently, both acidic and alkaline solutions can be used to etch aluminium surfaces during pre-treatment.
Alloying elements that affect corrosion
Besides the protective properties of the oxide layer, the corrosion resistance of aluminium alloys is determined by the presence of noble intermetallic particles. In the presence of an electrolyte solution, such as water or salt, corrosion can occur, with the noble particles acting as cathodes and the surrounding areas becoming anodes where the aluminium dissolves.
Even particles with small amounts of noble elements can exhibit high nobility due to selective dissolution of aluminium on their surfaces. Particles containing iron significantly reduce corrosion resistance, while copper also diminishes corrosion resistance. Higher concentrations of impurities, like lead, at grain boundaries also negatively impact corrosion resistance.
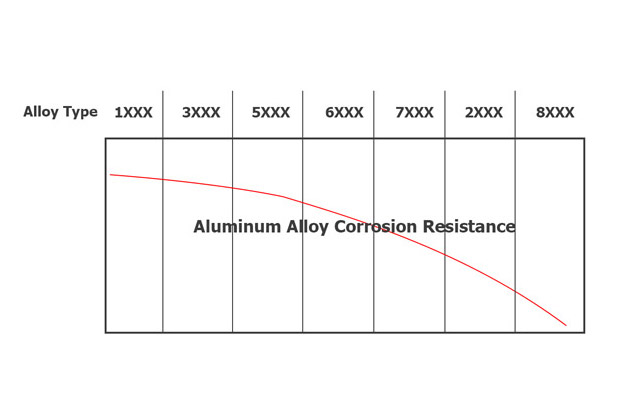
Corrosion resistance in 5000 and 6000 series aluminium alloys
Aluminium alloys from the 5000 and 6000 series generally have lower levels of alloying elements and intermetallic particles, resulting in relatively high corrosion resistance. High-strength 2000-series alloys, commonly used in the aviation industry, often have a thin cladding of pure aluminium to prevent corrosion.
Recycled alloys tend to contain increased levels of trace elements, making them slightly more susceptible to corrosion. However, the variation in corrosion resistance between different alloys, and even within the same alloy, due to production methods and heat treatments, can be greater than that caused by trace elements alone.
Therefore, it is crucial to seek technical knowledge from your supplier, especially if corrosion resistance is vital for your product. Aluminium is not a homogeneous material, and understanding its specific properties is essential in choosing the appropriate aluminium product for your needs.
Feel free to contact us if you want to know more.
Tel/WhatsApp: +86 17688923299 E-mail: aisling.huang@aluminum-artist.com
Post time: Oct-31-2023